New Clinical Endpoints in Primary Sjogren's Syndrome: An Interventional Trial based on stratifying patients

Funding body: Innovative Medicines Initiatives (IMI), Horizon 2020, EC/EFPIA
Dates: 2019 - 2024
Coordinator: INSERM, France
Consortium: 25 partners, 10 countries
Overall budget: €15.4 million
LBAI specific budget: € 389,986
Grant agreement No 806975
Website
The challenge:
Whereas 10 new targeted-immunomodulatory treatments have been marketed for rheumatoid arthritis in the past 20 years, only one drug has been licensed for other systemic AIDs, such as pSS and systemic erythematous lupus in the same period. There are several factors that may hamper the development of successful drugs for AID. Being multiorgan, these AIDs are considerably heterogeneous among individuals both in terms of clinical manifestations and biological disturbances, with, as a consequence, a great difficulty to set-up accurate composite clinical end-points sensitive to change and usable in clinical trials.
The objectives:
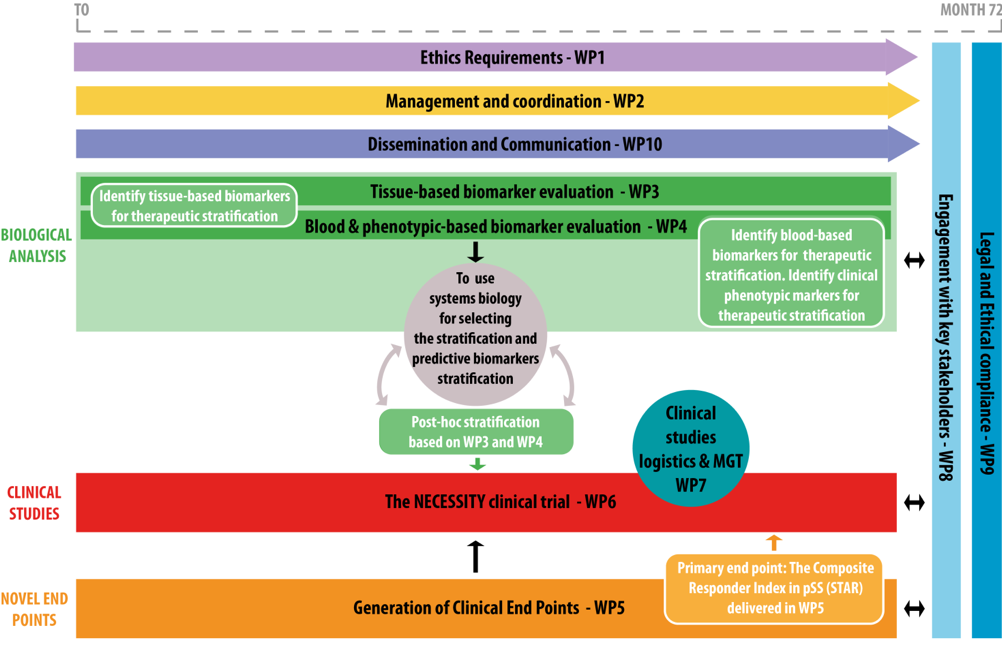
The NECESSITY project aims at developing new tools to accelerate the discovery of new treatments. It is built around several objectives:
1- To develop and assess sensitive clinical endpoints, for use in future clinical trials, able to evaluate response to drug treatments in patients with pSS with high disease burden and/or systemic involvement; 2- To identify and evaluate discriminative biomarkers for stratification of pSS patients predictive of organ involvement and disease progression and thus available for inclusion in clinical trials;
3- To set-up and perform an original multi-arm multi-stage clinical trial to validate the newly defined pSS endpoints and the identified biomarkers, by maximizing the chance of finding a difference between the placebo arm and the treated arm.
Expected results:
The impact of the NECESSITY project will be far reaching. New tools and methodologies will be available for industry to evaluate new drugs. Clinical care will be improved with a better understanding of the disease, a quicker diagnosis, and new therapies to treat and manage the disease. Healthcare delivery needs to support patients will be identified and will lead to the evolution of public policies. All these aspects will contribute to improving the quality of life of patients suffering from primary Sjögren’s syndrome.
LBAI involvement:
LBAI is involved in several Work Packages (WPs 3, 4 , 5 and 6), including in the development of new clinical endpoints (Pr Divi Cornec, WP co-leader) and in the stratification of patients (Pr JO Pers).
This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 806975. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.




