Taxonomy, Treatment, Targets and Remission. Identification of the Molecular Mechanisms of non-response to Treatments, Relapses and Remission in Autoimmune, Inflammatory, and Allergic Conditions.

Funding body: Innovative Medicines Initiatives (IMI), Horizon 2020, EC/EFPIA
Dates: 2019-2026
Coordinator: Fundación Pública Andaluza Progreso y Salud (FPS) (ES)
Consortium: 69 partners, 15 countries
Overall budget: €80 million
LBAI specific budget: €360,000
Grant agreement No: 831434
Project website
Presentation of the project
The challenge:
Autoimmune/inflammatory and allergic diseases are common, chronic, collectively impose a substantial healthcare burden and, while treatments are available, response and disease progression is unpredictable in individual patients.
Currently, too little is known about the mechanisms of non-response. So as to better predict response to treatment and potentially indentify new biomarkers leading to a better patients management and a personalised treatment, it is imperative to understand the molecular mechanisms underlying the development of each disease.
The objectives:
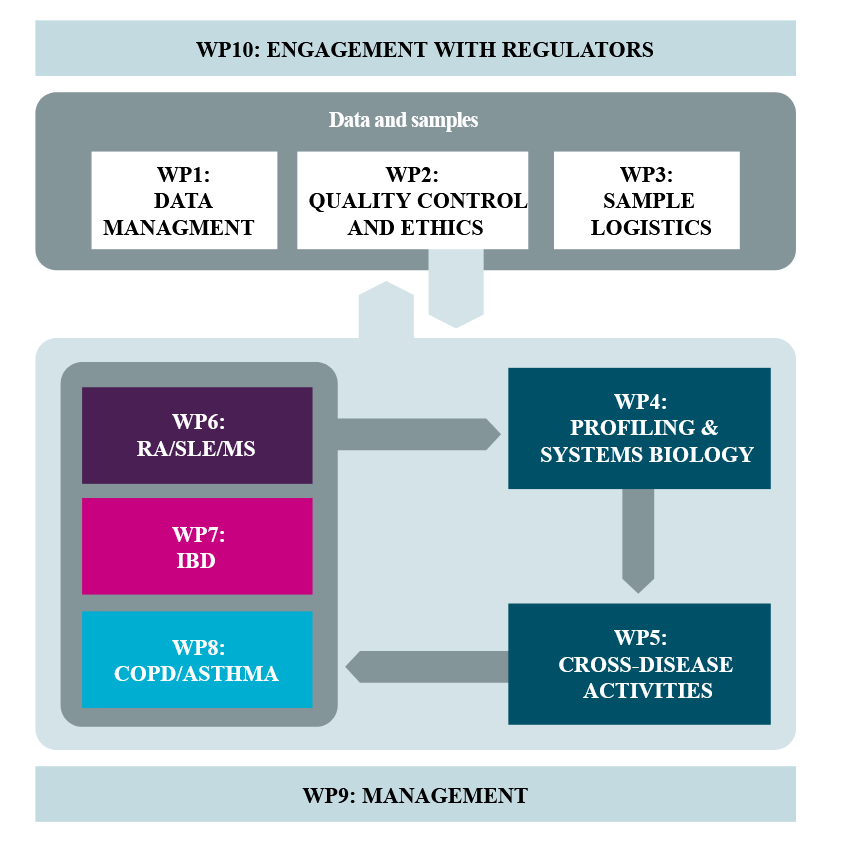
3TR is a highly integrated and transdisciplinary consortium that aims at improving knowledge of the molecular pathways and mechanisms related to response and non-response to treatment in seven different immune-mediated, allergic and inflammatory diseases: systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease, asthma and chronic obstructive pulmonary disease. Despite their heterogeneity, recent studies have shown that at the molecular level some groups of patients share certain characteristics of these diseases, suggesting that they may also share the pathways involved in response to treatment or disease progression.
The consortium of the project proposes multi-dimensional molecular analysis of multiple sample types collected longitudinally from homogeneously recruited patient cohorts suffering from seven different autoimmune, allergic and inflammatory diseases. This high-resolution multi-omics analysis of individualised disease trajectories will facilitate stratification and identification of molecular patterns that better predict response or non-response to therapy. A prospective longitudinal follow-up will include the de novo collection of multiple sample types from the same individual at multiple time points, 3TR will test a suite of molecular techniques to provide a comprehensive data foundation that will be analysed using advanced bioinformatics and modelling techniques. Furthermore, using a combination of data and samples from industry-sponsored clinical trials and academic studies harmonized with a new observational prospective trial collection of patients treated with standard of care therapy, 3TR major aim is to identify solid biomarker signatures as well as molecular pathways and mechanisms linked to response and non-response to therapy, always considering the different clinical dimensions of "response".
Expected results:
The methods will help to identify patients are likelihood to relapse/flare or enter remission. This detailed molecular analysis will serve as the basis for the development of integrated mechanistic/agent based models of the diseases. It will also aid the understanding of the processes in the target tissues and changes in the immune system, potentially providing a completely new approach to elucidating causal mechanisms; Taken together, this comprehensive approach will identify potential novel drug targets, opportunities for drug re-purposing and biomarkers. The autoimmune, allergic, and inflammatory disorders to be investigated, namely systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), multiple sclerosis (MS), inflammatory bowel disease (IBD), asthma, and chronic obstructive pulmonary disease (COPD), may share pathways of disease progression; therefore, we will study the seven diseases both in parallel and jointly. In this context, 3TR will have access to an unprecedented quantity of clinical data and samples of more than 50,000 patients across 50 clinical trials, ultimately aiming to discover and verify stratification biomarkers to improve patient management.
LBAI involvement:
LBAI is mainly involved in the analysis part of the project in link with imaging mass cytometry (WP4 and WP6).

This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement No 831434. The JU receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.




