Coordination Variations within Binuclear Copper Dioxygen-Derived (Hydro)Peroxo and Superoxo Species; Influences upon Thermodynamic and Electronic Properties.
P. K. Hota, A. Jose, S. Panda, E. M. Dunietz, A. E. Herzog, L. Wojcik, N. Le Poul, C. Belle, E. I. Solomon,* K. D. Karlin*
J. Am. Chem. Soc. 2024, in press, DOI: 10.1021/jacs.3c14422
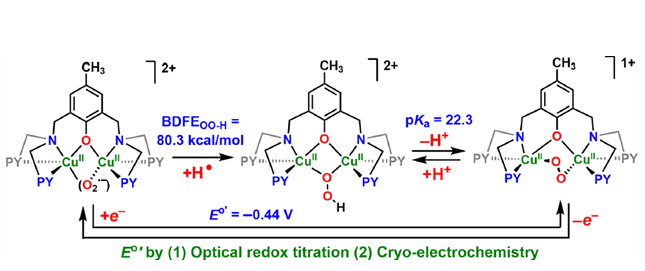
Copper ion is a versatile and ubiquitous facilitator of redox chemical and biochemical processes. These include the binding of molecular oxygen to copper(I) complexes where it undergoes stepwise reduction-protonation. A detailed understanding of thermodynamic relationships between such reduced/protonated states is key to elucidate the fundamentals of the chemical/ biochemical processes involved. The dicopper(I) complex [CuI2(BPMPO−)]1+ {BPMPOH = 2,6-bis{[(bis(2-pyridylmethyl)-amino]methyl}-4-methylphenol)} undergoes cryogenic dioxygen addition; further manipulations in 2-methyltetrahydrofuran generate dicopper(II) peroxo [CuII2(BPMPO−)(O22−)]1+, hydroperoxo [CuII2(BPMPO−)(−OOH)]2+, and superoxo [CuII2(BPMPO−)(O2•−)]2+ species, characterized by UV−vis, resonance Raman and electron paramagnetic resonance (EPR) spectroscopies, and cold spray ionization mass spectrometry. An unexpected EPR spectrum for [CuII2(BPMPO−)(O2•−)]2+ is explained by the analysis of its exchange-coupled three-spin frustrated system and DFT calculations. A redox equilibrium, [CuII2(BPMPO−)(O22−)]1+ ⇄ [CuII2(BPMPO−)(O2•−)]2+, is established utilizing Me8Fc+/Cr(η6-C6H6)2, allowing for [CuII2(BPMPO−)(O2•−)]2+/[CuII2(BPMPO−)(O22−)]1+ reduction potential calculation, E°′ = −0.44 ± 0.01 V vs Fc+/0, also confirmed by cryoelectrochemical measurements (E°′ = −0.40 ± 0.01 V). 2,6-Lutidinium triflate addition to [CuII2(BPMPO−)- (O22−)]1+ produces [CuII2(BPMPO−)(−OOH)]2+; using a phosphazene base, an acid−base equilibrium was achieved, pKa = 22.3 ±19 0.7 for [CuII2(BPMPO−)(−OOH)]2+. The BDFEOO−H = 80.3 ± 1.2 kcal/mol, as calculated for [CuII2(BPMPO−)(−OOH)]2+; this is further substantiated by H atom abstraction from O−H substrates by [CuII2(BPMPO−)(O2•−)]2+ forming [CuII2(BPMPO−)- (−OOH)]2+. In comparison to known analogues, the thermodynamic and spectroscopic properties of [CuII2(BPMPO−)] O2-derived adducts can be accounted for based on chelate ring size variations built into the BPMPO− framework and the resulting enhanced CuII-ion Lewis acidity.