M. Bourrez, F. Gloaguen. Electrochemical reduction and protonation of a biomimetic diiron azadithiolate hexacarbonyl complex: Mechanistic insights. Bioelectrochemistry 2023, 153, 108488-108495. DOI: 10.1016/j.bioelechem.2023.108488.
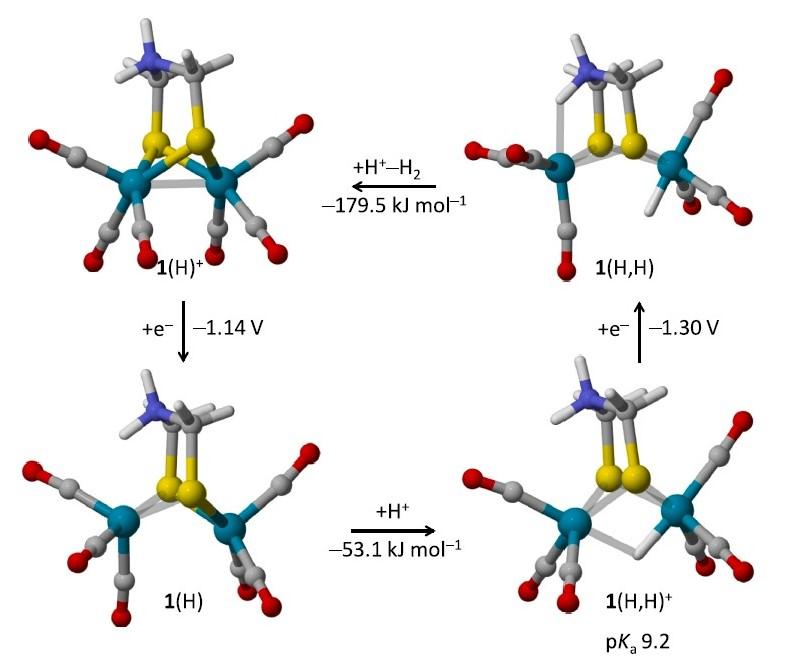
The electrochemical reduction and protonation of [Fe2(adtH)(CO)6] (1, adtH = SCH2N(H)CH2S) and [Fe2(pdt) (CO)6] (2, pdt = SCH2CH2CH2S) in the presence of moderately strong acid in acetonitrile was investigated by cyclic voltammetry (CV), focusing on the catalysis of hydrogen evolution reaction (HER) by a {2e ,2H+} pathway. The turnover frequencies at zero overpotential (TOF0) of the N-protonated product 1(H)+ and 2 for the HER were estimated from simulations of the catalytic CV responses at low acid concentration using a simple ECEC mechanism (two electrochemical and chemical steps). This approach confirmed that 1(H)+ is clearly a better catalyst than 2, pointing to a possible role of the protonable and biologically relevant adtH ligand in the enhancement of the catalytic performances. Density functional theory (DFT) calculations further suggested that, owing to a strong structural rearrangement in the course of the catalytic cycle, the HER catalysis by 1(H)+ only involves the iron center adjacent to the amine group in adtH and not the two iron centers as in 2. Since terminal hydride species (FeFe H) are known to more easily undergo protonolyse to H2 than their bridging hydride isomers (Fe H Fe), this may explain here the enhanced activity of 1(H)+ over 2 for the HER.