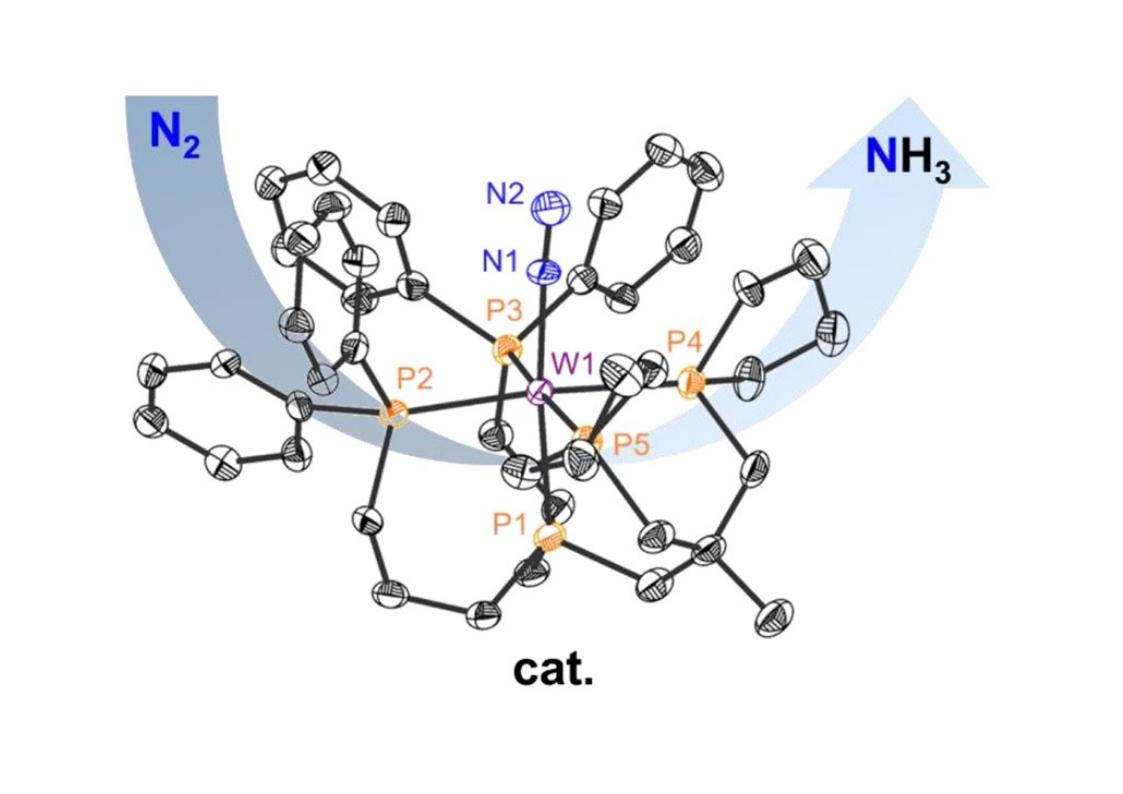
Chemocatalytic Conversion of Dinitrogen to Ammonia Mediated by a Tungsten Complex.
A.-M. Vogt, T. A. Engesser, J. Krahmer, N. Michaelis, M. Pfeil, J. Junge, C. Näther, N. Le Poul, F. Tuczek,
Angew. Chem. Int. Ed. 2024. DOI: 10.1002/anie.202420220
Whereas molybdenum dinitrogen complexes have played a major role as catalytic model systems of nitrogenase, corresponding tungsten complexes were in most cases found to be catalytically inactive. Herein, we present a modified pentadentate tetrapodal (pentaPod) phosphine ligand in which two dimethylphosphine groups of the pentaPodMe (P5Me) ligand have been replaced with phospholanes (Pln). The derived molybdenum complex [Mo(N2)P5Pln] generates 22 and the analogous tungsten complex [W(N2)P5Pln] 7 equivalents of NH3 from N2 in the presence of 180 equiv.s of SmI2(thf)2/H2O, rendering the latter the first tungsten complex chemocatalytically converting N2 to NH3. In contrast, the tungsten complex [W(N2)P5Me] generates ammonia from N2 only in a slightly overstoichiometric fashion. The reasons for these reactivity differences are investigated with the help of spectroscopic and electrochemical methods.